
Author Affilications:School of Mineral Processing and Bioengineering, Central South University
Abstract: The abundant resource of steel slag makes it an interesting prospect for long-term COstore through mineral carbonization . In this paper, the dissolution of steel slag in acetic acid (HAc) solution and the immobilization of COin in steel slag leachate are investigated to synthesize high-value CaCO. The results showed that the microwave water bath conditions were beneficial for increasing the leaching rate of Ca and Mg elements in steel slag, while the low concentration HAc solution showed high selectivity for Ca+Mg. In microwave-enhanced water bath reactions, the leaching rate and selectivity of Ca+Mg can be increased by increasing the reaction temperature, extending the leaching time, decreasing the concentration of the HAc solution, and increasing the stirring rate. The kinetic parameters of elemental calcium, including the apparent activation energy, were 14.51 kJ⋅mol, indicating control by surface chemical reactions. Finally, by introducing 20% CO into the leachate, high-purity CaCO whiskers could be synthesized at 400 W ultrasonic conditions at a reaction temperature of 70 °C, a reaction time of 15 minutes, and a solution pH of 9.1. This strategy promotes the parallel utilization of thin smoke gases and large quantities of solid steel slag waste, thereby improving the economic and environmental performance of the steel industry.
Keywords: steel slag microwave enhancement leaching kinetics fixed whisker CaCO
Summary: Since CO emissions play an important role in driving climate change, the increase in CO emissions, combined with the temperature rise of about 1.0°C to 1.5 °C since pre-industrial times, has become an urgent global problem [1–2]. However, burning fossil fuels such as coal, oil, and gas for energy production, transportation, and industrial activities remains a major source of CO emissions [3,4]. Disturbingly, the continuous release of carbon dioxide from the atmosphere has led to the greenhouse effect, which has led to global warming, which has negative impacts on ecosystems and human health [5–6]. Therefore, it is imperative to explore and advance efficient technologies to reduce CO2 emissions.
CCUS (Carbon Capture, Utilization, and Storage) technology is a key carbon reduction technology that aims to capture and utilize CO while storing it safely for long periods of time, effectively reducing emissions into the atmosphere. Despite this, traditional CCUS technologies face challenges such as high cost, geological storage constraints, and safety concerns during transportation [7–11]. To overcome these obstacles, emerging methods have been proposed, such as the carbonization of calcium-magnesium minerals and waste residues. Carbonization of calcium-magnesium minerals involves the natural reaction of magnesium and calcium-rich minerals with CO, resulting in the formation of stable carbonate compounds. This technology not only allows for long-term storage of CO, but also facilitates the conversion into high-value mineral products, such as CaCO with different particle sizes and morphologies[12]. On the other hand, industrial solid wastes containing calcium and magnesium can permanently sequester carbon dioxide emissions from industrial processes through mineralization. This approach not only reduces waste emissions and dependence on natural resources, but also reduces CO2 emissions by converting waste residue into valuable products. For example, CO emitted from steel mills can chemically react with the calcium and magnesium minerals found in steel slag waste. This reaction ultimately results in a durable carbonate product while addressing the problems associated with the instability of steel slag and limited resource utilization.
The carbonization methods of steel slag mainly include direct method and indirect method. The direct method involves the direct reaction of CO with steel slag to form carbonate compounds. However, this method requires high temperatures and pressures, resulting in large energy consumption, complex operations, and slow reaction rates. In contrast, indirect methods involve the introduction of leaching agents, such as strong acids [13–16], weak acids [17–20], or ammonium salts [21,22], in a solution environment to extract calcium and magnesium from steel slag. A calcium-magnesium-rich solution is then utilized to sequester CO and produce carbonate products. Indirect methods provide milder reaction conditions, simpler handling, easier control of carbonation products, and higher carbonation efficiency. Theoretically, 240-580 kg of CO per ton of steel slag could be potentially immobilized[23-25]. However, there are current challenges in the leaching process, including the high cost of leaching agents, poor selectivity for leaching of various elements in steel slag, and the difficulty of achieving complete leaching using the weak acids associated with ammonium salt leaching agents.
Hong et al. [26] used organic acids extracted from biomass waste as enhancers (e.g., HAc, propionic acid, butyric acid, and valeric acid) to improve the efficiency of metal extraction from steel slag. They explored the integration of two waste streams to achieve the overall sustainability of the carbonation technology. The results show that organic acids have a higher extraction efficiency for elements in steel slag than strong acids, and the organic acid mixture has the highest extraction efficiency, indicating that ligands with different size and stability constants have a synergistic effect. However, increasing the concentration of organic acids leads to reduced selectivity for calcium and magnesium, while decreasing concentrations results in higher selectivity but lower extraction efficiency [27,28]. Therefore, it is valuable to explore ways to improve the leaching efficiency of calcium and magnesium ions at low concentrations of organic acids while maintaining their selectivity.
In recent years, microwave technology, as a special heating method that is green and environmentally friendly, has made significant progress in the field of mineral leaching. Olubambi et al. [29] studied the leaching behavior of complex sulphide ores with the assistance of microwaves. They found that the presence of microwave fields increased surface cracks and reduced ore strength, resulting in higher leaching rates. Krishnan et al. [30] investigated the effect of microwave-assisted leaching on zinc extraction from tailings and observed that the zinc leaching rate exceeded 90% within 6 min under microwave conditions, which was significantly higher than that under traditional water bath conditions. Microwave-assisted heating offers unparalleled advantages over traditional water bath heating, such as heat transfer to the minerals and selective element leaching [31]. Microwave heating can quickly heat polar solvents, promote leaching reactions, increase the leaching rate of precious metal elements, and reduce reaction time and energy consumption.
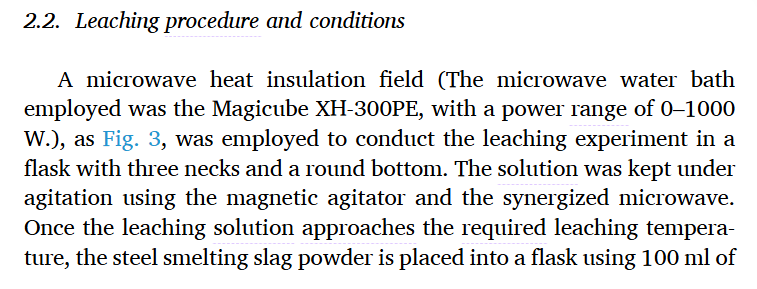
In this manuscript, we describe a steel slag carbonization technique that uses acetic acid (HAc) as the leaching solvent. In addition, we studied in depth the effects of different experimental parameters on the slag leaching process in a microwave environment. It reveals the kinetic mechanism of calcium leaching in steel slag and synthesizes high-value CaCO products by adsorption of low-concentration CO in leachate. The process combines the selective leaching capabilities of HAc with the uniformity and efficiency provided by microwave-enhanced heating. As a result, the calcium and magnesium elements are selectively separated and leached from the steel slag. It solves the challenges of unstable steel slag properties and limited resource utilization, and shows broad development prospects. In addition, the extracted solution can be used for COsequestation and the synthesis of high-purity, high-value carbonate materials. This has greatly contributed to the advancement of CCUS technology in the steel industry, while generating significant social and economic advantages.
The use of Xianghu instruments in this article:
Conclusion:
In a microwave water bath reaction field, the leaching kinetics of Ca in steelmaking slag were studied using a green solvent as HAc. Preliminary XRD, SEM-EDS, and particle size analyzer detection of the raw sample ensured the presence of Ca, Fe, Mg, and Al in the main phase, microscopic appearance, and particle size distribution in the slag. Then, the leaching behavior of various elements in the leaching process of steel slag, the kinetic mechanism of calcium leaching, and the synthesis of whisker-type CaCOin in the leaching solution were deeply studied, and the following findings were obtained.
(1). In contrast to conventional heating, microwave heating causes thermal stress fractures between minerals in steel slag, increasing particle breakage and leaching rates of calcium and magnesium.
(2). Under the conditions of 0.5 M HAc, the reaction temperature is 60 °C, the reaction time is 30 min, the stirring rate is 300 r/min, the solid-liquid ratio is 25 g/L, and the microwave power is 600 W.
(3). Aluminum-calcium minerals are retained in the leaching resources, while the iron components are retained in the form of calcium oxide, magnetite or a small amount of mafic minerals, so as to improve the resource utilization.
(4). The leaching kinetics of calcium activation energy is 14.51
The principle of kJ⋅mol strongly supports the mechanism of chemical control of the surface.
(5). When the CO concentration in the flue gas is 20%, the curing temperature is 70 °C, the reaction time is 15 min, the ultrasonic reaction field is 400 W, and the steel slag leaching solution can adsorb CO to synthesize whisker-like CaCO. The process effectively resulted in a permanent fixation of 276 kg of CO, resulting in 607 kg of high-value CaCO product per ton of steel slag.
